Clintouch
Supporting patients with serious mental illnesses via mobile phones.

Current management of serious mental illness is limited, partly because symptom assessment is intermittent and conducted during consultation with clinicians who ask service users to recall symptoms up to a month earlier.
This has led to problems with averaging and biased recollection, which makes accurate assessment difficult.
The ClinTouch app circumvents these difficulties and was developed for use on Android smartphones to capture symptom data in real-time, once or several times daily. Technical development and field testing were funded by the Medical Research Council.
Investigating the use of a mobile phone to support service users with psychosis
During test phases service users have found ClinTouch both feasible and acceptable.
Using a mobile phone minimises intrusion into the daily lives of service users, with no need for an extra device. ClinTouch enables automatic data transfer allowing data to be monitored in real-time.
A monitoring system for clinicians can be used as an adjunct to enhance psychosocial intervention by prompting discussion during consultations, tracking medication effects, detecting relapse signatures and allowing clients to see personal progress or triggers occurring prior to relapse
Key Features of the ClinTouch Software
- intelligent programming reduces the number of questions posed by branching questions according to the severity of earlier answers ;
- touch-based user interface;
- users can click or drag scale to answer questions using an analogue slider;
- two semi-random alarms per day;
- multiple question sets.
Results
When the ClinTouch app was confirmed to an SMS based solution and with "gold standard" (PANSS and CDS) clinical assessments we found:
- good connection between ClinTouch and "gold standard" interview ratings;
- ClinTouch assessments took less time to complete;
- comparison between ClinTouch and SMS showed better compliance with ClinTouch;
- patients preferred ClinTouch to SMS based system.
Expected benefits of ClinTouch Technology
- enhanced quality of life for those with psychosis, a long term condition, through prevention of Deliberate Self Harm (DSH) and relapse;
- targeted help for people who are recovering from an acute exacerbation of ill health;
- improved self-management;
- better collaborative care;
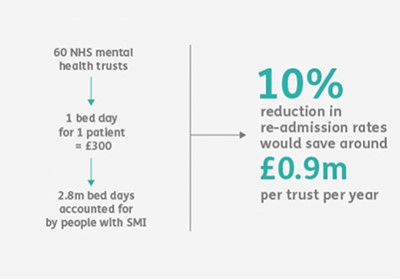
- cost savings (reducing unscheduled admissions to hospital).
Further Development: CareLoop
The team are refining the software and clinical protocols in response to feedback; building a fully functional end-to-end solution for remote symptom monitoring embedded in NHS trust care services, providing continual updated diagnostic information for clinical decision making.
Aims
- to design, build and deploy the interface for clinical teams;
- involve service users in making ClinTouch highly personalised;
- build in optional self-management components, including medication management, side effect monitoring and negative symptom assessments;
- carry out a randomised, controlled trial to evaluate how effective the system is at preventing relapse.
Social networking support will also be explored.
We are using qualitative research and working with service users and carers, as well as the charity, 'Rethink' Mental Illness, in order to ensure the user experience is central to the design of the system.
Future development will:
- refine the existing application and question content;
- develop clinician interface and algorithm detecting risk of relapse and self-harm;
- build end-to-end solutions used to enhance patient care;
- trial in 2 NHS trusts (MMHSCT and SLaM) with 80 participants;
- develop a business strategy for deployment;
- extend into psychological treatment delivery, relevant biosensor development, social networking functions and medication management.
Manchester Connected Health Ecosystem Partners Involved
ClinTouch development and testing is funded by three Medical Research Council grants.
It is undertaken by The University of Manchester and Manchester Mental Health and Social Care Trust, with the Manchester Academic Health Sciences Centre.
Since 2012, all phases of development have been shaped by the active involvement of service users from MMHSC Trust.
The University of Manchester and Manchester Mental Health and Social Care Trust conducted the trial with 70 Schizophrenia patients including acute, remitted and ultra-high risk patients.
The project, funded by the Medical Research Council, ran from November 2010 to October 2012 and continues with further MRC funding.
